The Birth of Hydrated Electrons from Aqueous Iodide
The first study modeling the mechanism of hydrated electron formation from aqueous iodide
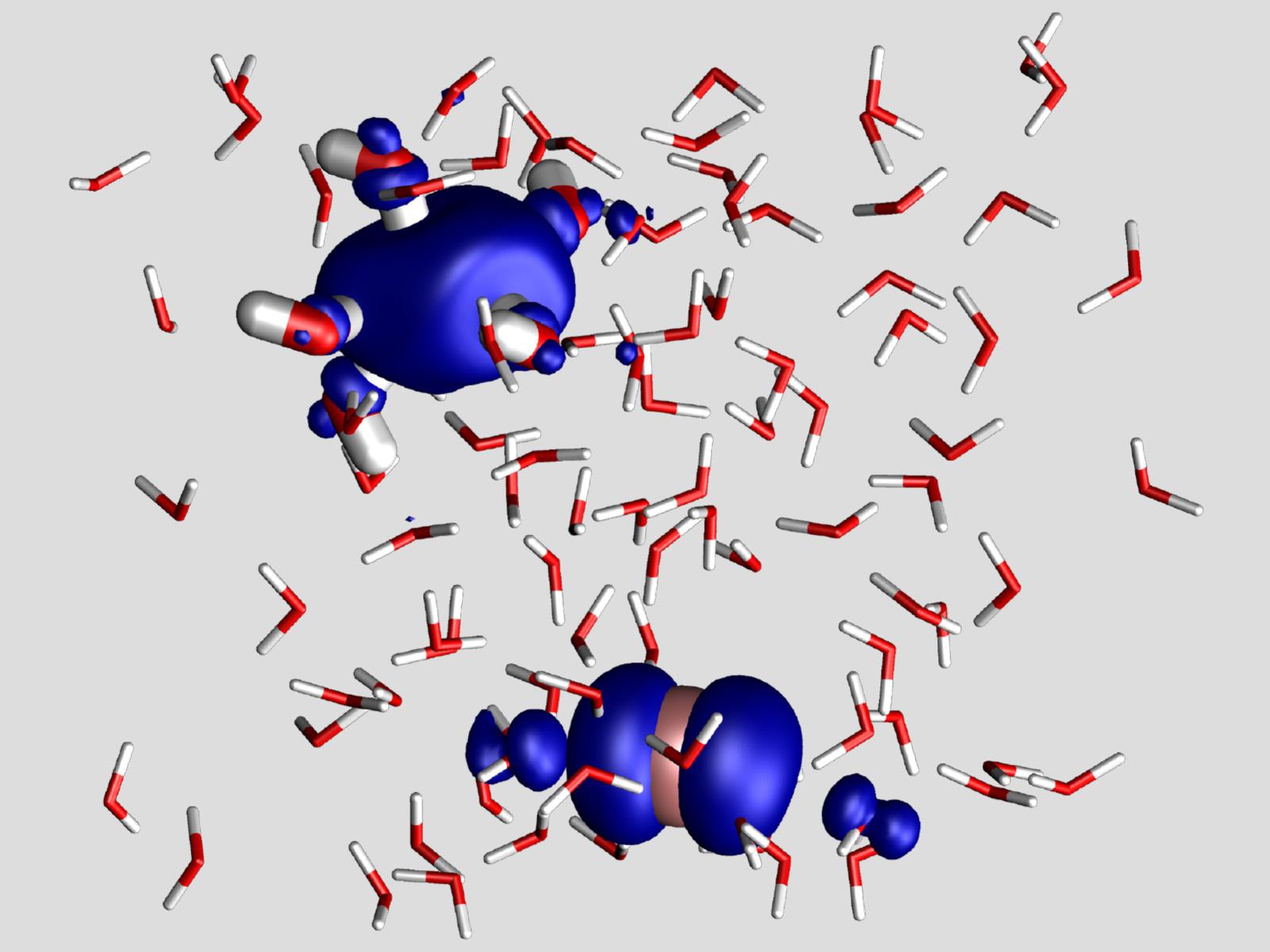
Understanding how hydrated electrons form can help researchers model radiation-induced electron transfer processes.
(Image by Britta Johnson | Pacific Northwest National Laboratory)
The Science
When researchers need electrons in solution, they commonly use the radiation-induced decomposition of iodide. However, scientists had yet to model exactly how this process happens on a molecular level. New work used three sets of density functional theory simulations to follow the mechanism of generating hydrated electrons from iodide for the first time. The team found that the electron begins as “trap-seeking,” forming at voids within the water. After initial formation, the electron moves to a single location that is stabilized by the solution.
The Impact
Hydrated electrons generated from aqueous iodide are used in a wide range of chemical reactions. This work identifies the mechanism by which these electrons form at the earliest stages, a previously unstudied regime. In addition to providing valuable insight on the iodide reaction, this modeling approach could be used to simulate other radiation-induced electron transfer processes in solutions.
Summary
In a laboratory setting, hydrated electrons are often generated by exciting an aqueous ion, such as I-(aq), to a charge-transfer-to-solvent (CTTS) state. Researchers conducted the first ab initio molecular dynamics simulation of the spontaneous generation of the hydrated electron via this CTTS state. They modeled the mechanism using three different density functional theory protocols. Each protocol was used to simulate a different portion of the mechanism by which the solvated electron is generated. The CTTS state was initialized using information from various ground state structures. By tracking the variability in the position of the initially excited electron, the researchers concluded the solvated electron is initially “trap-seeking” and preferentially forms at voids in the water structure. Once the frustrated I-*(aq) forms, the electron quickly localizes and is rapidly stabilized by the solvent in “trap-digging” behavior.
Researchers used the results from the simulations to construct a model for the electron generation mechanism. They fit the potential energy for the ground I-(aq) and the equilibrated e-(aq)states to Gaussian curves with the electrostatic potential of the solvent water molecules serving as the collective reaction coordinate. As the solvent reorganizes, the CTTS state is stabilized and the energy decreases. The CTTS to solvated electron relaxation can occur adiabatically via pure solvation dynamics, suggesting that solvent reorganization may limit the electron transfer rate.
PNNL Contact
Gregory Schenter, Pacific Northwest National Laboratory, greg.schenter@pnnl.gov
Funding
K.C.-F. acknowledges support from the Department of Energy (DOE) Office of Science (SC), Office of Workforce Development for Teachers and Scientists, SC Graduate Student Research (SCGSR) program. The SCGSR program is administered by the Oak Ridge Institute for Science and Education (ORISE) for the DOE. ORISE is managed by ORAU under Contract Number DE-SC0014664. J.M.H. acknowledges support from the National Science Foundation (Grant No. CHE-1955282). B.A.J, C.J.M., and G.K.S. are supported by the DOE SC, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences, and Biosciences. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of DOE, ORAU, or ORISE. Calculations were performed on both Pacific Northwest National Laboratory’s Institutional Computing resource, the National Energy Research Scientific Computing Center (NERSC), and the Ohio Supercomputer Center. NERSC is a DOE SC user facility located at Lawrence Berkeley National Laboratory, operated under Contract No. DE-AC02-05CH11231.
Published: September 19, 2023
Carter-Fenk K., B. A. Johnson, J. M. Herbert, G. K. Schenter, and C. J. Mundy. 2023. “Birth of the Hydrated Electron via Charge-Transfer-to-Solvent Excitation of Aqueous Iodide,” J. Phys. Chem. Lett., 14, 870. [DOI: 10.1021/acs.jpclett.2c03460]