Legacy Research
Editor's Note: The IDREAM research described here represents the scientific focus from 2016 to summer 2024.
Science Thrust 1: Molecular and Solution Processes
Lead: Zheming Wang
Goal: Understand the solvent dynamics, solute–solvent organization, prenucleation species, and radiation-driven reactivity in concentrated alkaline electrolytes.
Solution-phase dynamics play a crucial role in the origin of the physical properties of fluids and influence kinetically hindered steps toward precipitation and dissolution. For aqueous Al(III) chemistry in basic solutions, these range from the local perturbation of Al(OH)4- coordination geometry and aggregation into dimers and oligomers to the formation of ion-pairing networks and cluster densification. These structures become especially evident in highly concentrated "water-in-salt" solutions, where there is not enough water present to fully solvate each ionic species.
Task 1.1: Quantify the driving forces behind long-range solution organization and assembly of prenucleation species in highly organized ion–ion networks in the absence of ionizing radiation.
Current research in concentrated alkaline solutions focuses on understanding solution organizational structures. We seek to determine how the presence of variable cation and anion species promotes the formation of local polyhedral arrangements, ion–ion networks, and the perturbation of the bulk water tetrahedral structure. These organizational structures are anticipated to significantly alter the chemical reactivity of these materials and lead to distinct reaction pathways specific to these extremely alkaline conditions.
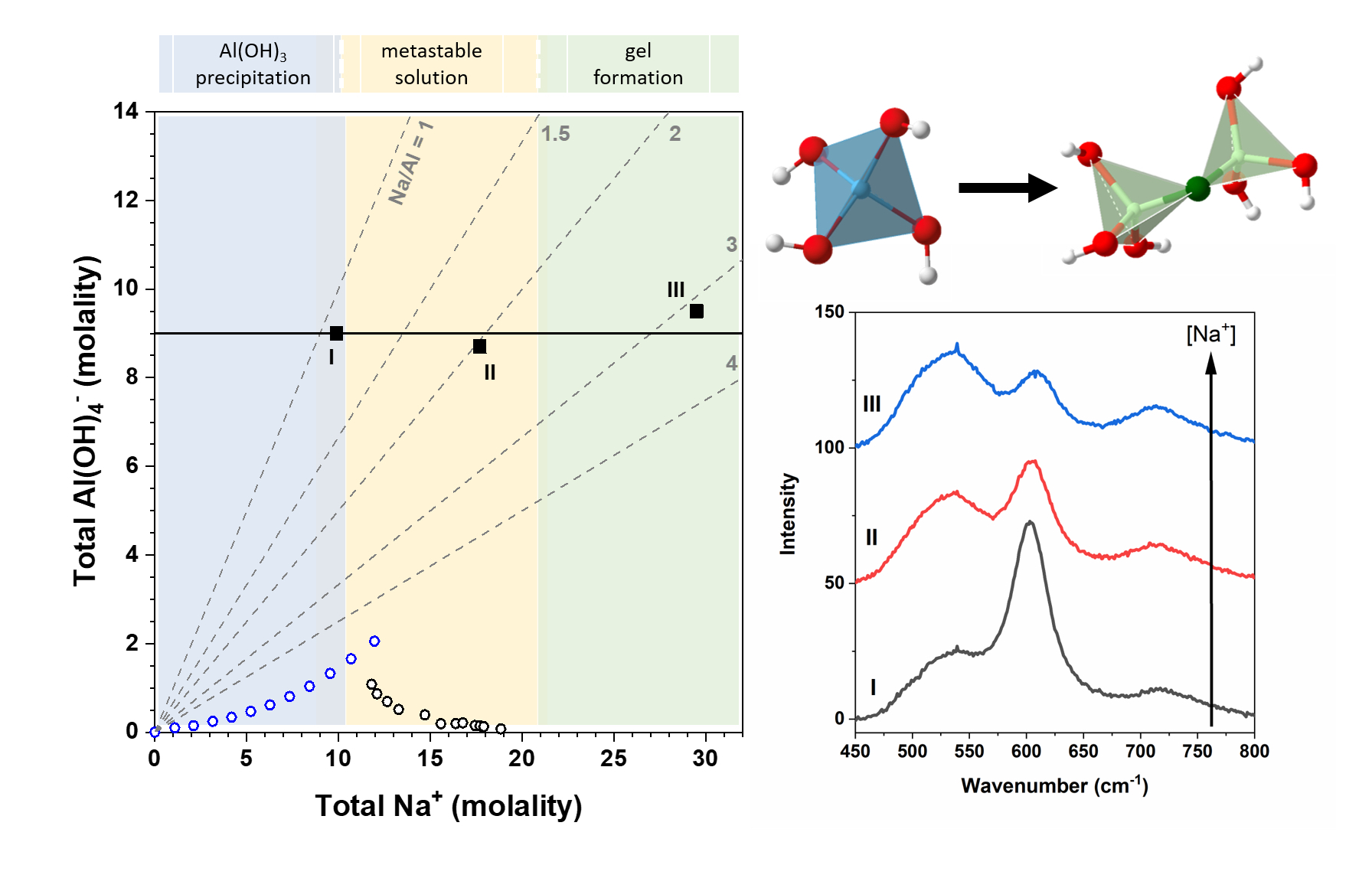
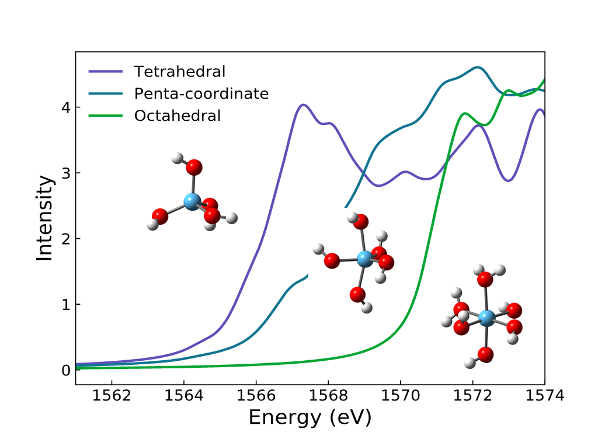
In studies of alkaline sodium aluminate solutions, we are probing local aluminate structures, such as aluminate monomers and dimers, and how they relate to the solutions’ susceptibility toward gibbsite precipitation or enhanced metastability (Figure 1). Such knowledge will help unravel the underlying mechanism by which the tetrahedrally coordinated aluminate solution species transforms into the octahedrally coordinated aluminum in gibbsite.
These solutions are being investigated with geometrical fitting of neutron and X-ray pair distribution functions (PDFs). The aluminate structures used in the geometrical fitting were obtained through ab initio and classical molecular dynamics (MD) simulations, featured within the IDREAM Cross-Cutting Theme 2 (XC2). Supplementing these studies, we have employed Raman and Fourier transform infrared (FTIR) spectroscopies to quantify the relative abundances of aluminate monomer and dimer species. Through these studies, it has become evident that hydrogen bonding and proton transfer reactions greatly influence the aluminum speciation and metastability of these solutions. The role of hydrogen bonding is being further investigated through studies comparing the effects of substituting deuterium for protium.
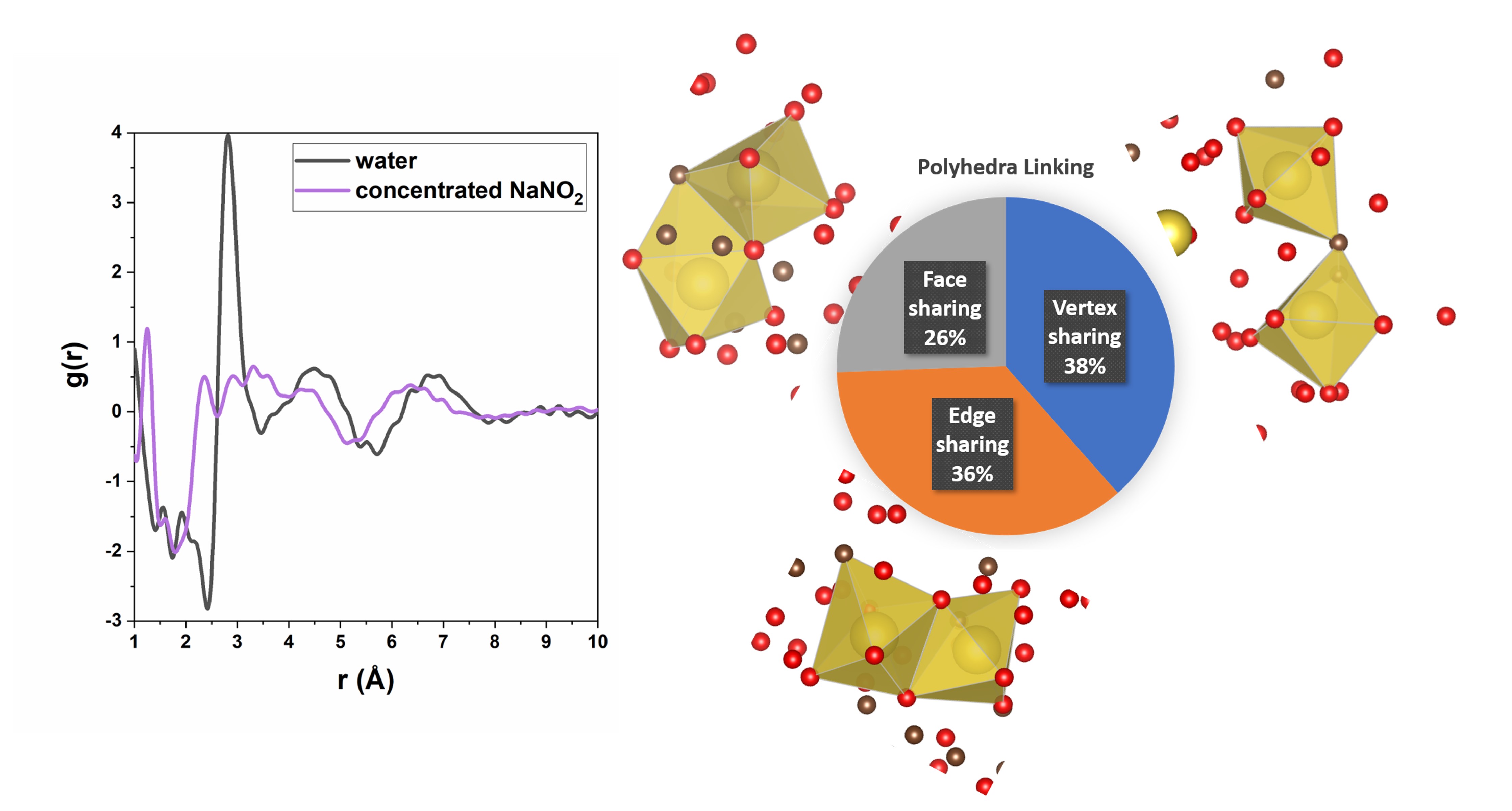
In addition to probing the local structures in sodium aluminate solutions, long-range solution structures are being investigated in systems containing alkali nitrates and alkali nitrites. These solutions have been investigated through X-ray PDF analysis, small angle X-ray scattering, Raman spectroscopy, and FTIR spectroscopy. MD simulations (XC2) are being performed to investigate the complex O‧‧‧O correlations from approximately 3–8 Å in the PDF, as observed initially in our earlier work on solutions containing NaNO2 and NaOH. Initial MD results for concentrated NaNO2 reveal that solutions at these length scales reflect a polyhedral network consisting of nitrite anions, Na+, and water. As the concentration of the solution increases from undersaturation conditions to saturation, this network of ions becomes increasingly connected. We seek to connect the solution structures to the observed differences in solubilities between the various alkali nitrates and nitrites, as well as to the increase in solubility in their mixtures.
Task 1.2: Discover the radiation-driven chemical dynamics governing the formation of nonequilibrium steady-state species and their role in prenucleation speciation.
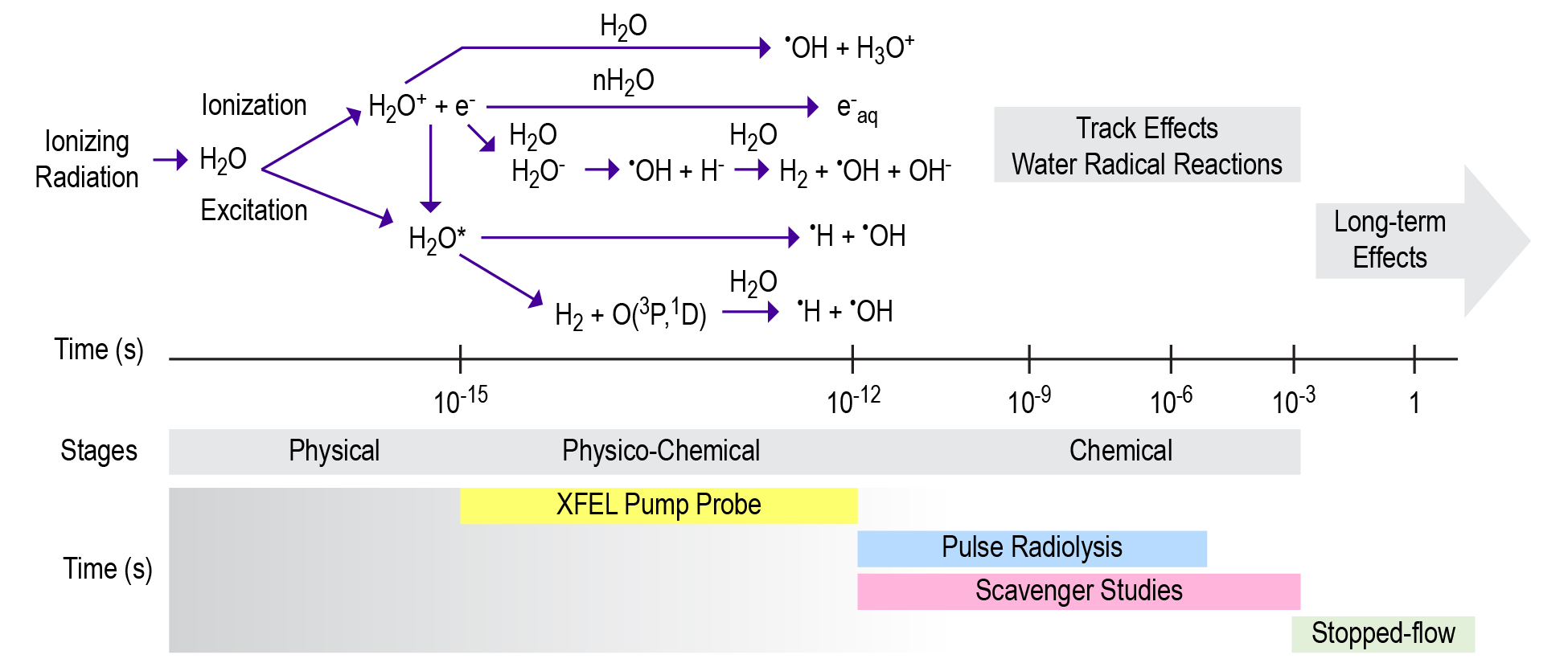
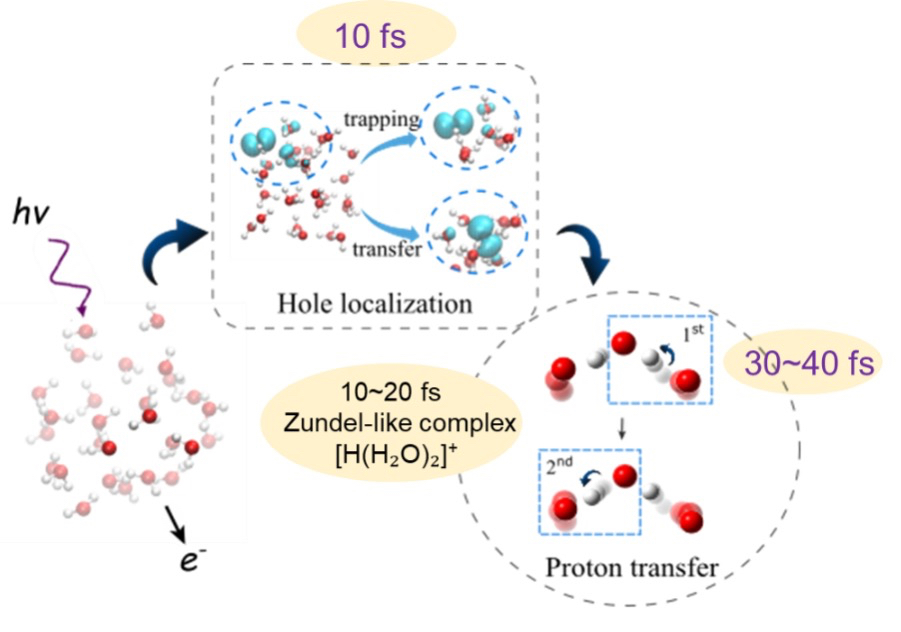
Current efforts to understand the effects of radiation on concentrated solutions spans multiple timescales (Cross-Cutting Theme 1 [XC1]: Radiolysis and Radiation Dynamics) that can be subdivided into stages of radiolysis (Figure 3). A focus of IDREAM is to determine the processes occurring in the physico-chemical and chemical stages to understand the underlying mechanisms controlling the long-term effects on materials exposed to ionizing radiation, such as H2 gas production, precipitation, and aggregation behaviors.
Initial studies are unraveling the formation and reactivity of chemically reactive species in the radiolysis of water on the physico-chemical timescale using X-ray pump–probe experiments at the Linac Coherent Light Source. Following the investigations on water, the effect of solute species such as NaOH, NaNO2, and NaNO3 will be delineated. These species not only allow an additional experimental probe (such as Na k-edge or N k-edge) but also allow us to look at the effects of radiolysis and the subsequent reaction pathways when water is not necessarily the next nearest neighbor. These ultrafast radiolysis experiments benefit from a comprehensive computational team. Ab initio Ehrenfest dynamics simulations (XC2) are being utilized to determine the ultrafast processes following ionization in bulk water and aqueous electrolytes based upon the solution structures determined through ab initio and classical MD signatures in Science Thrust 1 Task 1.1, and X-ray absorption near edge structure signatures are being computed for potential species that form after ionization.
Science Thrust 2: Interfacial Structure and Reactivity
Lead: Kevin Rosso
Goal: The research proposed for Science Thrust 2 (ST2) is focused on understanding chemical transformations that lead to the precipitation of solids such as gibbsite, boehmite, and aluminate salt hydrates, as well as the dynamics of processes occurring at interfaces with low-water, highly alkaline solutions.
Task 2.1: Identify the nucleation and precipitation processes driving interface formation under water-limited conditions.
ST2 is steadily unraveling mechanisms governing nucleation and growth during the precipitation and crystallization of aluminum (oxy)hydroxide and alkaline aluminate salts shown in the phase diagram in Figure 6. Recent work has focused on identifying and understanding the roles of metastable intermediates that emerge during the crystallization of aluminum-bearing phases in acidic, circumneutral, and alkaline conditions. Under acidic to circumneutral conditions, relict oligomeric aluminum hydroxide clusters, in which the aluminum coordination is six-coordinate, were recently identified as inclusion defects in gibbsite nanoplatelets through X-ray scattering analysis, with the molecular structure of the cluster constrained by supporting magic angle spinning nuclear magnetic resonance spectroscopy, Raman spectroscopy, and Fourier transform infrared spectroscopy. The retention of liquid-state motifs of aluminum oligomer motifs was observed in alkaline conditions during the crystallization of sodium aluminate hydrates under alkaline conditions. That is, the sodium mu-oxo aluminate dimer was observed for the first time with magic angle spinning nuclear magnetic resonance spectroscopy.
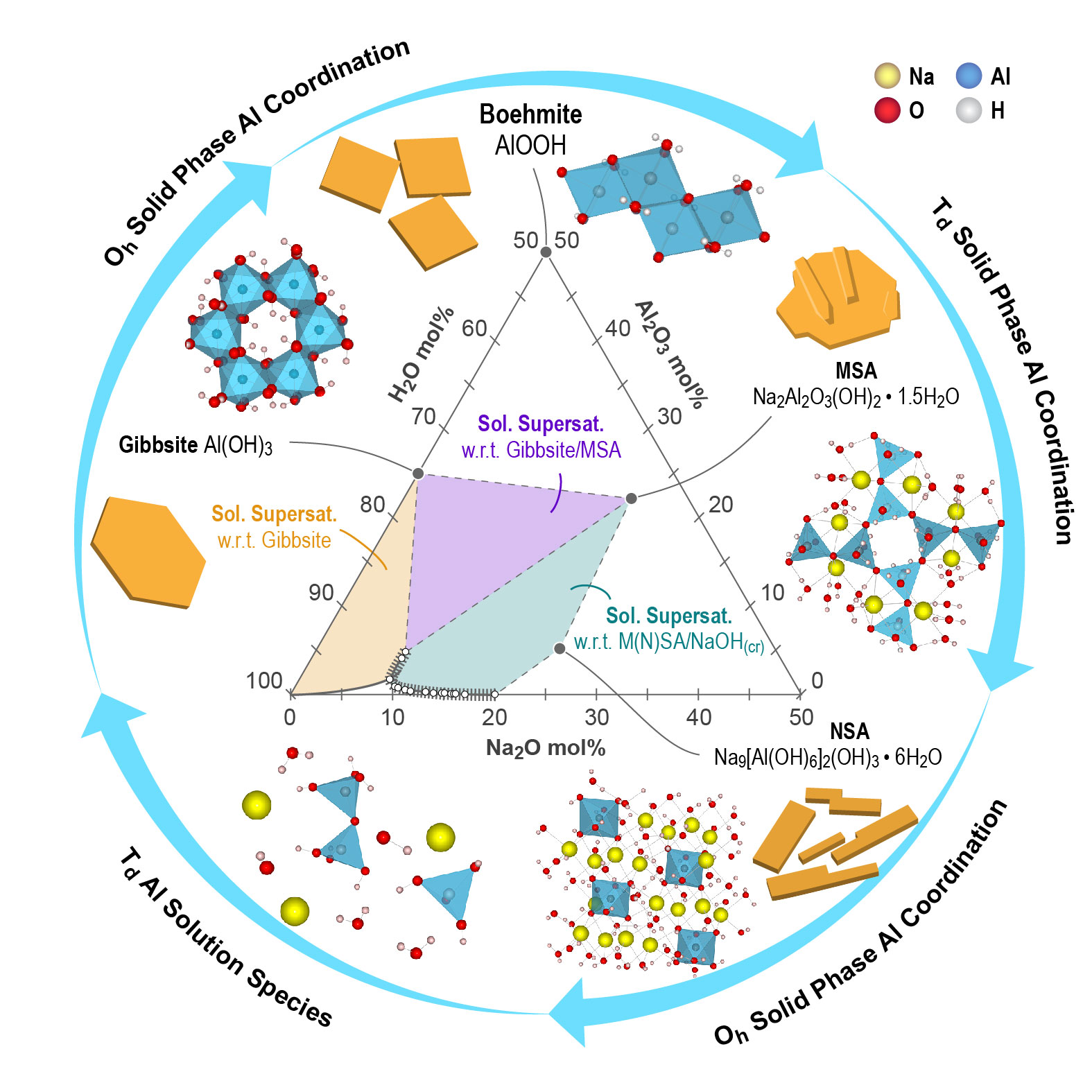
Ongoing research is building on these observations to probe for similar intermediates during aluminum hydroxide crystallization mechanisms in alkaline conditions. An aspect of this exploration is examining the effect of varying alkaline cations in the MOH solutions from which the aluminum hydroxide precipitates, where M+ = Li+, Na+, K+, Rb+, and Cs+, to understand how nucleation may depend on water availability controlled in part by the hydration dynamics of the alkali cation. These studies will build upon recent work tracking the sensitivity of transformations between potassium aluminate salts and aluminum hydroxide in the form of gibbsite or dawsonite to the partial pressure of carbon dioxide, as well as on studies investigating the variation of the crystalline phase between gibbsite and the potassium mu-oxo aluminate dimer related to variations in system composition. These studies are intrinsically linked to those in IDREAM Science Thrust 1 (ST1), where the effects of cation identity on solution organization are also under investigation.
Part of understanding aluminum coordination change mechanisms at interfaces includes examining the dissolution of aluminum oxyhydroxides. ST2 is exploiting high-resolution atomic force microscopy (AFM) and high-precision synthetic gibbsite nanoplatelets (Cross-Cutting Theme 3 [XC3]: Synthesis and Materials Characterization) to probe dissolution mechanisms in situ at the atomic scale in highly alkaline solutions, complemented by computational molecular simulations (Cross-Cutting Theme 2 [XC2]: New Computational Tools and Theory) (Figure 6). In situ AFM on the basal (001) surface shows single-layer etch pit nucleation and growth defined by near simultaneous retreat of (100) and (110) step edges in aqueous NaOH solutions, suggesting similar aluminate detachment mechanisms at these two crystallographically distinct step edges. In our first generation of molecular simulations, we mapped the free-energy landscape of aluminate detachment from (110) step edges and found that the overall reaction combines kink formation and kink propagation. Two individual reactions were found to be rate-limiting for kink formation, namely, the displacement of Al from a step site to a ledge adatom site and its detachment from ledge/terrace adatom sites into the solution. As a result, a pool of mobile and labile adsorbed species, or adatoms, exists before the release of Al into solution. Because of the quasi-hexagonal symmetry of gibbsite, kink site propagation can occur in multiple directions at similar rates. Ongoing molecular simulation work will examine both (100) and (110) step edges to both test this hypothesis and also seek quantitative agreement in predicted etch pit opening rates compared to those observed through AFM.
In addition to these activities, work is also underway to expand upon recent studies that discovered Cr(III) cluster formation during sorption on boehmite and a consequential decrease in boehmite dissolution kinetics. In addition to the presence of trivalent cations, changes in the solubility of aluminum hydroxides and other salts due to dissolved nitrogen oxyanions, such as nitrite and nitrate, are under investigation to better understand how ion–ion interactions in mixed electrolytes influence solubilities. Such work begins to approach the complexity of high-level nuclear waste in real-world scenarios, where processing is not only limited by the immense compositional variation in the stored waste but also by the presence of radiation fields.
Task 2.2: Quantify the impact of ionizing radiation in driving nucleation, growth, and dissolution.
Studies of the effects of ionizing radiation in conjunction with Cross-Cutting Theme 1 (XC1) on nucleation, growth, and dissolution have begun to unveil the molecular-level basis for how radiation-driven changes at interfaces alter phase stability and reactivity. X-ray, energetic photon, and electron irradiation can ionize and electronically excite target atoms and molecules. These excitations undergo complicated relaxation and energy-transfer processes that ultimately determine the manifold system responses to the deposited excess energy. For example, in weakly bound gas- and solution-phase samples, intermolecular Coulomb decay (ICD) and electron-transfer-mediated decay (ETMD) can occur with neighboring atoms or molecules, leading to efficient transfer of the excess energy to the surroundings. In ionic solids such as metal oxides, intratomic and interatomic Auger decay produces localized final states that lead to lattice damage and typically the removal of cations from the substrate. The relative importance of Auger-stimulated damage (ASD) versus ICD and ETMD at microsolvated nanoparticle interfaces is not well known and is an important aspect of ST2 research.
In our studies of electron bombardment of microsolvated boehmite, essentially no lattice damage resulting from the ionization and electronic excitation could be detected. Instead, efficient energy transfer and soft ionization of interfacial water molecules were observed. This is likely a general phenomenon at gas–oxyhydroxide nanoparticle interfaces where the density of states of the ionized chemisorbed species significantly overlaps with the core hole states of the solid. To examine this process in more detail, ST2 and XC1 studied the production and release of H2 and O2 during and after electron (100–1000 eV) irradiation of boehmite nanoplatelets. H2 and O2 produced during irradiation likely correspond to electron-induced dissociation of the hydroxyls primarily in the terminal surface layers of the boehmite followed by combinative desorption and abstraction reactions. Post-irradiation temperature-programmed desorption shows a temperature-dependent release of H2 but not O2, which likely involves diffusion and recombination of hydrogen atoms initially trapped between the boehmite layers followed by recombination at edge and surface sites. H2 production upon thermal annealing of irradiated material shows no dependence on flux, suggesting the precursor hydrogen atom species trapped within the bulk are long lived.
By contrast, ST2 studies using gamma irradiation indicate that trapped electrons and related O-centers are major radiolytic products. Gibbsite powders and their thermally dehydrated forms were rehydrated and irradiated with gamma rays to examine the effects of the collapse of the crystalline gibbsite structure as it is converted to alumina on the transport of precursors of molecular hydrogen to the surface. The amount of hydrogen gas produced from irradiated gibbsite and its transition phases with adsorbed water increased substantially with increasing temperature of dehydration of the samples. H2 production was considerably lower for samples without water than the hydrated samples, signifying the importance of surface water and the transport of precursors to the surface. Electron paramagnetic resonance spectroscopy showed that, in this case, the major radiolytic products are trapped electrons and related O-centers.
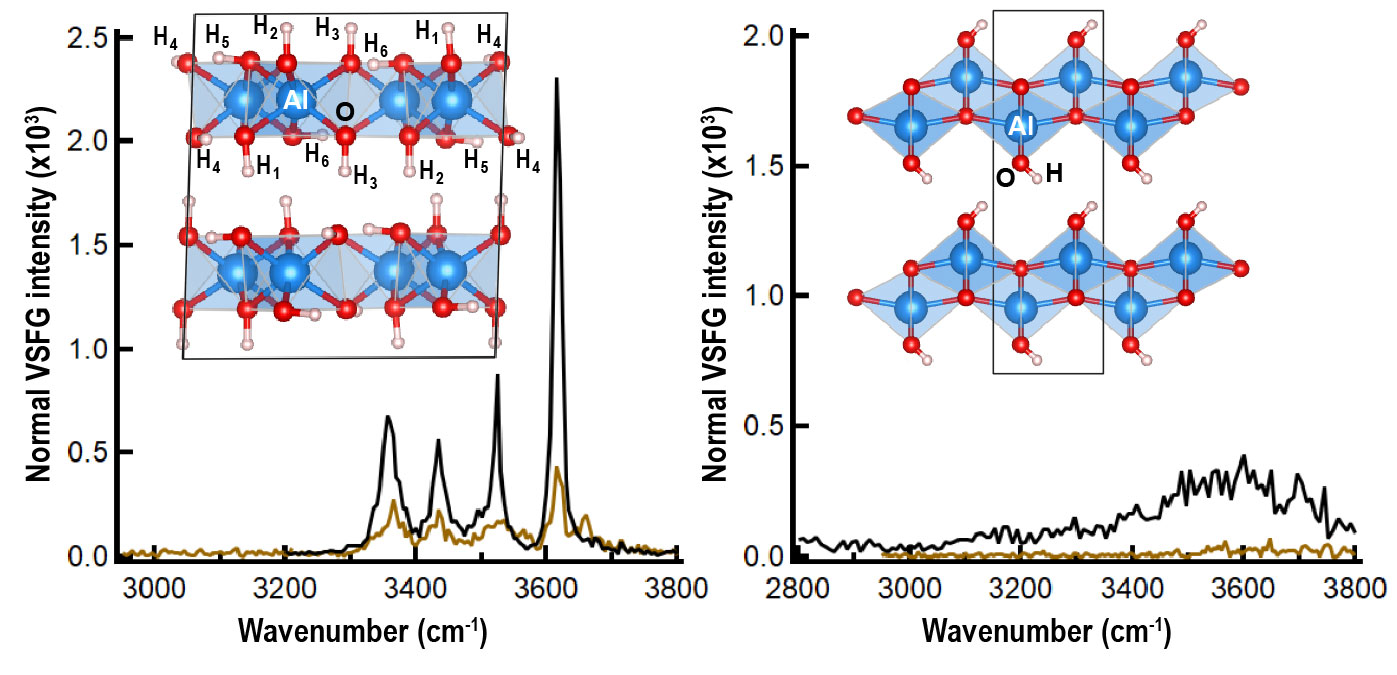
Given the importance of water at interfaces to radiolysis, significant investments in the characterization of hydrated interfaces with vibrational sum-frequency-generation spectroscopy have provided interface-specific information on how structural hydroxyl groups and adsorbed water are modified by ionizing radiation (Figure 7). Gamma irradiation causes substantial scission of surface hydroxyl groups both on gibbsite and boehmite basal surfaces. Intriguingly, rehydration of gamma-irradiated boehmite and gibbsite interfaces was observed to recover the hydroxyl density on gibbsite but not boehmite surfaces. Additional characterization of the gibbsite and boehmite nanoparticles using electron paramagnetic spectroscopy indicated that radiolysis products such as oxygen radicals and/or F-centers in gibbsite and boehmite persist over many months. This work characterizing the interface in radiation fields is foundational to studies underway to quantify the effects of radiation on dissolution and precipitation processes via a novel RadAFM equipped for in situ irradiation of solid–liquid interfaces by X-rays. Given the complexity of dissolution and precipitation processes in the absence or presence of radiation, a key component of the strategy is to guide the analysis of experimental observations with XC2 MD simulations, as described in the cross-cutting themes.
Science Thrust 3: Dynamics of Confined Electrolytes
Lead: Andrew Stack
Goal: Understand the chemical and radiation-driven phenomena of nanoscale-confined electrolytes that lead to interactions between interfaces to form aggregates of particles.
Macroscale properties of slurries originate in part from the molecular interactions that occur between particles. In ST3, we are focused on understanding the structure and dynamics of alkaline electrolytes confined between particle surfaces. Current research is focused on determining the roles of interfacial water, hydroxide, dissolved impurities, and solutes confined between solid surfaces in controlling interparticle forces and discover how interfacial radiolysis affects these interactions.
To probe the interactions between particles and confined fluids that drive particle interactions in high-salt environments, ST3 builds on knowledge generated in ST2 about interfacial structure and reactivity and on that in ST1 about solution structure and ion–ion organization in highly concentrated alkaline electrolytes. Advancement in our understanding of confined electrolytes at the nanoscale and their influence on particle interactions will be accelerated through our Cross-Cutting Themes focused on radiation dynamics (XC1), new theory development (XC2), and materials synthesis (XC3).
Task 3.1: Discover the molecular-scale influence of particle interactions on aggregation in the absence of radiation
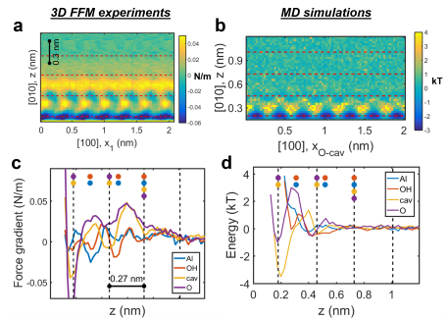
Current state-of-the-art science tools and expertise developed by IDREAM are being leveraged to probe particle geometry and electrolyte composition effects in situ. These include a complementary combination of amplitude-modulated AFM-based 3D fast force mapping (3D FFM) to determine interfacial structures (Figure 8), AFM–dynamic force spectroscopy to directly measure interaction forces, and the dynamics of neutron and X-ray scattering and nuclear magnetic resonance of particle slurries in highly concentrated and low-water environments. These experimental approaches are interpreted by and used to constrain simulations of particle aggregation in an iterative process using a combination of atomic-scale simulations and modified colloid theory of particle aggregation. These simulations include rare event methods (metadynamics) in conjunction with classical MD force fields (ClayFF) to observe the free energy of particle attachment under varying conditions in silico (XC2), with the goal of unraveling how interface composition (e.g., electrolyte composition, including the water–salt ratio) and interfacial structure affect aggregation.
Task 3.2: Understand the impact of interfacial radiolysis on the dynamics of particle interactions.
Radiation fields can change bulk solution (e.g., ionic strength, pH, and speciation) and surface chemistries (e.g., surface charge distributions), leading to significant impacts on particle interactions. Currently, synchrotron, AFM, and transmission electron microscopy (TEM) based techniques are being utilized to measure irradiation effects both ex situ and in situ. Synchrotron-based in situ small-angle hard X-ray (≥10 keV) scattering measurements simultaneously generate radical species and small-angle scattering to measure particle distributions, which are compared to X-ray free small-angle neutron scattering (SANS) experiments to quantify the effects of radical species on aggregation. Tumbler- and rheo-(ultra)small-angle X-ray scattering (U)SAXS)/SANS measurements under radiation (i.e., X-ray beam) extend these results to show ensemble averages of aggregation behavior. Scattering results demonstrate that boehmites exposed to gamma radiation have different aggregation behavior than pristine materials. To obtain direct observations of aggregation behavior in the presence of a radiation field, a combination of complementary liquid-phase TEM (LP-TEM) and cryo-TEM complements the scattering-based studies (Figure 9). The combination of LP-TEM and cryo-TEM simulates the effects of radiation on particle dynamics and interactions by modulating the dose of the electron beam in LP-TEM and minimizing the effect of the electron beam in cryo-TEM. In the same fashion, AFM dynamic force spectroscopy of irradiated boehmites directly measures interaction forces at the nanoscale between irradiated particles. This technique is complemented by our specialized rad-AFM capability, which enables measurements of particle interactions within a radiation field. Again, to connect these experimental observations with atomic-scale structure, dynamics, and reactivity, these experimental techniques are interpreted with computational methods such as rare event classical MD simulations, which are used to understand how oxygen and aluminum vacancies on the surfaces of boehmite particles affect interfacial and confined fluid structure. These approaches, along with modified colloid theory (based on Derjaguin Landau, Verwey & Overbeek [DLVO] theory), will establish unprecedented knowledge of correlations between radiation, chemistry, and particle interactions over different length scales due to transient species and provide the opportunity to compare results with those from Task 3.1 where radiation is absent.

Cross-Cutting Themes
In addition to the three science thrusts, IDREAM will continue to drive discoveries through the following cross-cutting themes:
- Cross-Cutting Theme 1: Radiolysis and Radiation Dynamics (Lead: Thomas Orlando)
- Cross-Cutting Theme 2: New Computational Tools and Theory (Lead: Aurora Clark)
- Cross-Cutting Theme 3: Synthesis and Materials Characterization (Lead: Xin Zhang)