Technology Overview
Human coronaviruses are emerging pathogens that can cause significant, global morbidity and mortality. The Middle East respiratory syndrome coronavirus (MERS-CoV), which emerged in 2012, has caused approximately 2,400 cases with a ~35% mortality rate in 27 countries—and transmissions continue to occur. To date, seven strains of human coronaviruses have been identified, including the severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2), which is the causative agent of COVID-19. Currently, no U.S. Food and Drug Administration-approved, drug-based treatment is available for MERS-CoV-infected patients.
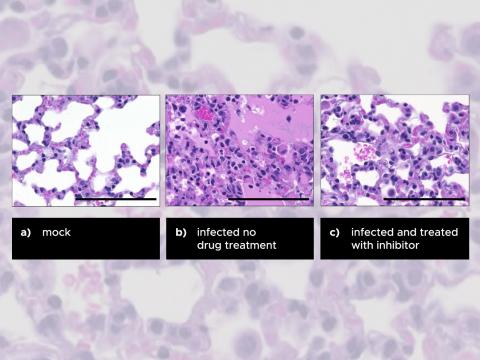
In the wake of a global coronavirus pandemic, comprehensive experimental approaches are needed that target how MERS causes tissue damage in order to reverse severe disease outcomes. After analyzing thousands of measurements of proteins, molecular messengers, and other signals that occur after infection, PNNL pinpointed the PERK 44 inhibitor that may help reduce mortality in coronavirus-infected patients. Future research could explore whether this method can be used to treat infections and diseases caused by other human coronaviruses.
Whereas other proposed treatments target MERS-CoV viral genomic RNA replication, this method treats coronavirus infection by targeting a host pathway that MERS-CoV harnesses to enhance infection. In particular, this inhibitor targets a host pathway that appears to be critical for virus replication and, as a result, reduces both levels of virus replication and burdens of disease. In animal models of MERS-CoV infection, which recapitulate the disease outcomes seen in humans, treatment with the inhibitor improved respiratory function while reducing lung tissue damage and weight loss. This inhibitor may work against other human CoV that can cause severe disease.
Licensees can administer this method alone or in combination with one or more other antiviral agents to treat a particular condition. Effective doses of the agents of this method can be administered in different compositions, either simultaneously, separately, or sequentially.
Advantages
- Provides methods for treating diseases and disorders associated with coronavirus infection.
- Can be used to treat infections and diseases caused by human coronaviruses.
- Targets a host pathway that appears to be critical for virus replication.
State of Development
While initially designed for treatment of coronavirus, this method could be applicable to a broader range of viruses and identifies an important mechanism for symptom management and progression.