Promoting Dihydrogen Activation at Copper for Catalytic Hydrogenation
New trigonal planar copper alkyl and hydroxide complexes promote distinct mechanisms for hydrogen activation, enabling the catalytic hydrogenation of challenging substrates without the use of precious metals
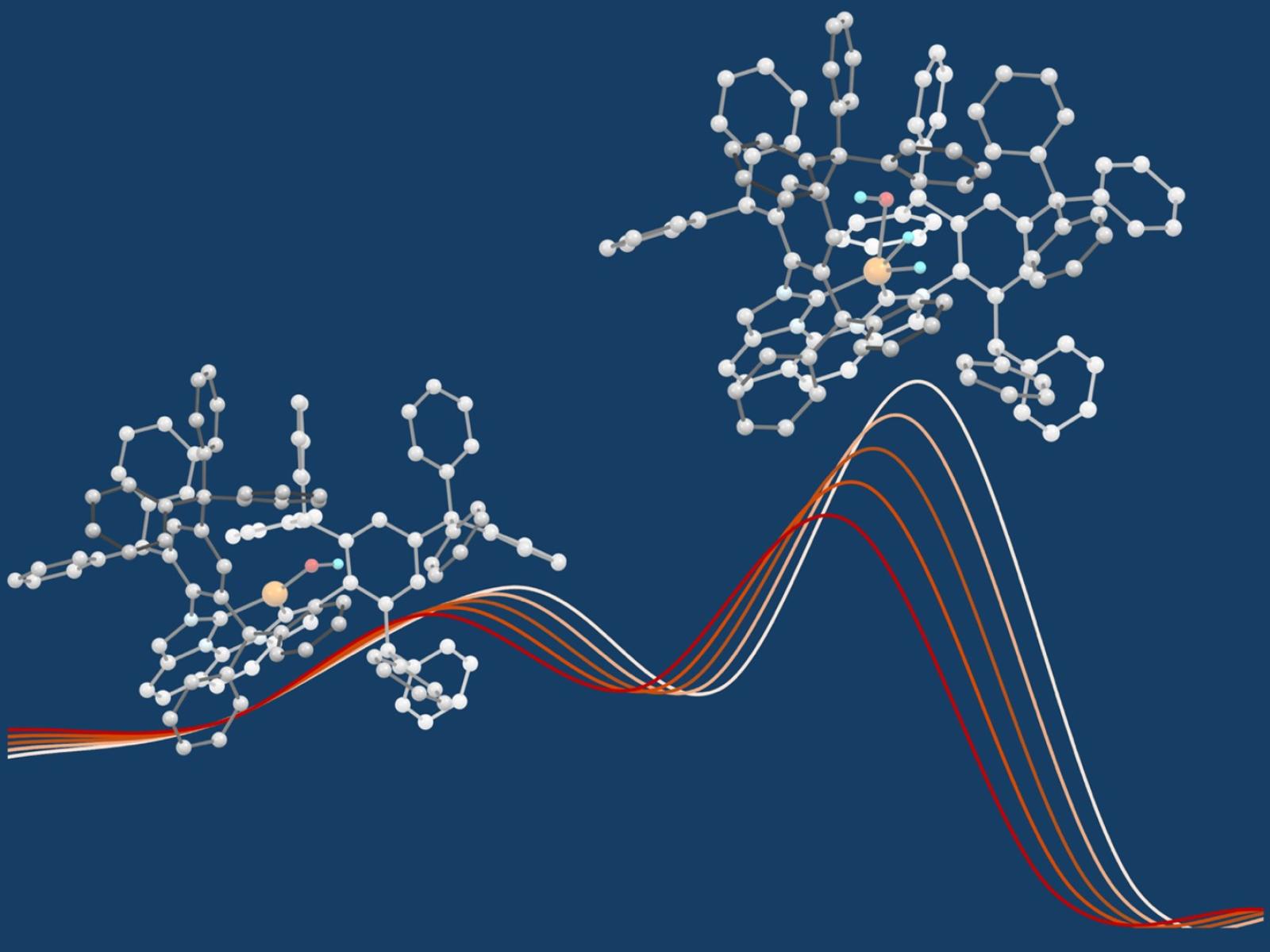
The splitting of a dihydrogen molecule at a protected single copper-hydroxide active site.
(Image by Ba Tran | Pacific Northwest National Laboratory)
The Science
Copper-based catalysts are commonly used for hydrogenating molecules with more reactive bonds, but reaction with less reactive molecules is rare. Researchers investigated the steps of hydrogenation on less reactive olefins with copper hydride (CuH) complexes geometrically tuned through ligand design. They found that trigonal Cu alkyl and hydroxide complexes were reactive toward dihydrogen, whereas the corresponding linear complexes were not. A combination of experimental kinetic and theoretical studies provides mechanistic insight into the key H2 activation step under mild temperature and pressure.
The Impact
Precious metal catalysts generally dominate olefin hydrogenation in industrial applications. This study shows that geometric control at a single-site copper center enables Earth-abundant metal catalysis of unactivated olefins at mild reaction conditions, expanding options for hydrogenation beyond precious metals. The work highlights key mechanistic insights and design strategies for activating H2 with base metals.
Summary
Molecular copper hydrides (CuH) are prominent base-metal catalysts capable of inserting into a variety of unsaturated substrates. Olefin insertion into CuH is common, but subsequent catalytic olefin hydrogenation has been inaccessible due to the high temperature and pressure needed for H2 activation at a single-site Cu(I). The precise mechanism of the H2 activation step has been difficult to study because the resulting CuH species can become unreactive because of aggregation. Researchers designed a new biscarbene ligand that forced the on-cycle Cu(I) hydride species to maintain a trigonal planar geometry, rendering the CuH more reactive to olefin insertion and providing kinetic stability against aggregation. They were able to isolate and spectroscopically examine the complex in situ during catalysis. Combining the experimental work with computational studies revealed key details of the unique mechanism of H2 activation that enables completion of this catalytic cycle under mild conditions. This work demonstrates the hydrogenation of unactivated olefins—a substrate class typically restricted to precious metal catalysis.
The results of this study show the impact of the synergy and close collaboration in the Institute for Integrated Catalysis between synthetic chemists, kineticists, computational chemists, and X-ray crystallographers to address knowledge gaps in molecular CuH catalysis.
Contact
Ba Tran, Pacific Northwest National Laboratory, ba.tran@pnnl.gov
Zdenek Dohnalek, Pacific Northwest National Laboratory, Zdenek.Dohnalek@pnnl.gov
Funding
This work was supported by the Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, Catalysis Science program, FWP 47319.
Published: August 19, 2025
Patrick, E. A., Sharada, S. M, Zoraster, A., Erickson, J. D., Ryan, D. E., Bullock, R. M., and Tran, B. L. 2025. "Trigonal Planar Bis(carbene)Cu(I) Complexes Enable Divergent H2 Activation with H2O for Accelerated Olefin Hydrogenation." Angew. Chem. Int. Ed., 147, e202510627. [DOI: 10.1002/anie.202510627]