Measuring Proton Diffusion in Supercooled Water
The first measurement of the proton diffusion constant at cryogenic temperatures provides insights into the mechanism of proton movement in supercooled water
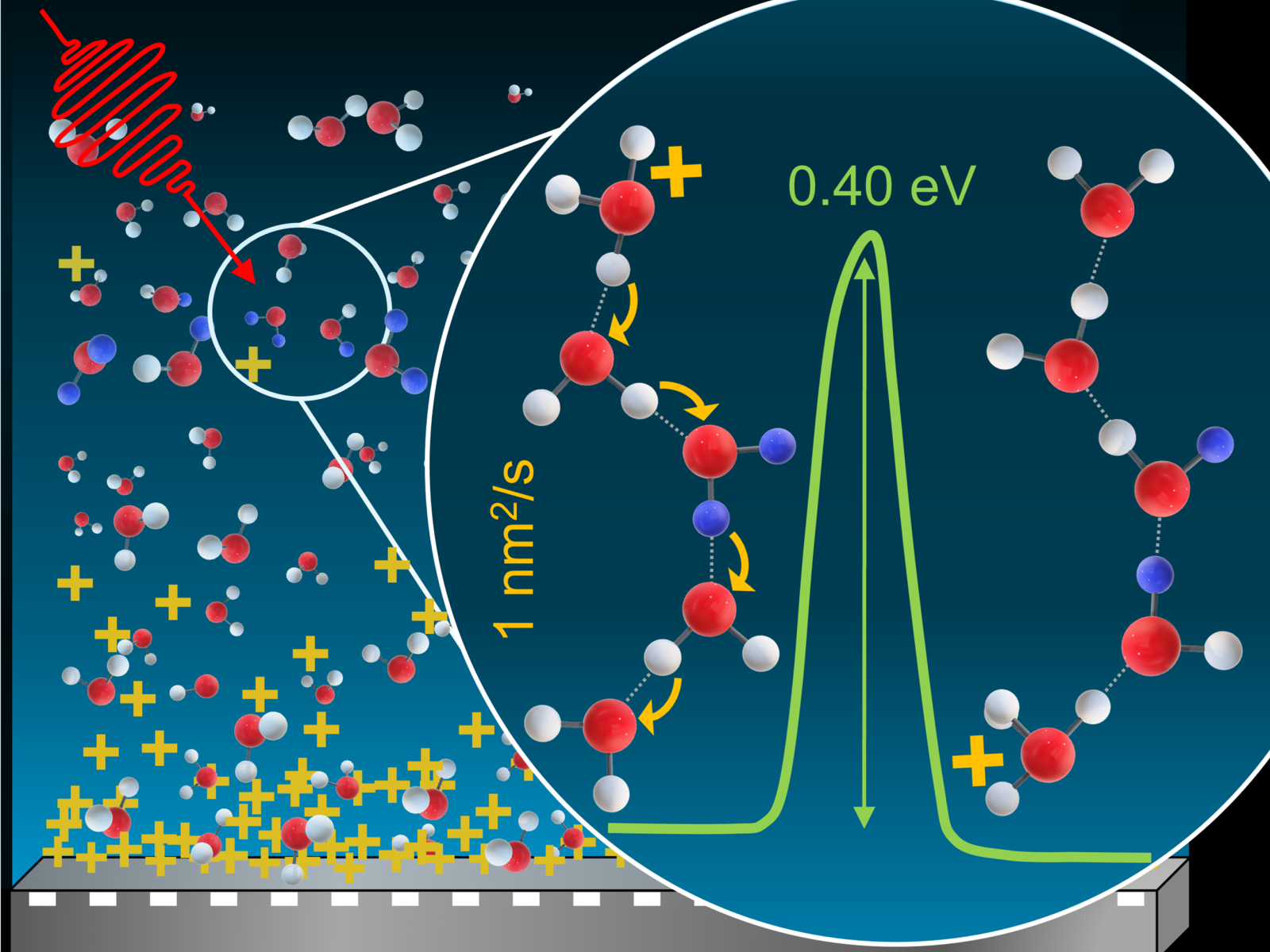
Hydrogen–deuterium exchange data provide information about the spatial distribution of protons within amorphous solid water, the energy barrier required for proton diffusion, and the proton diffusion coefficient.
(Image by Megan Dunlap | Pacific Northwest National Laboratory)
The Science
Water is everywhere and acts as the primary solvent for biological processes. Despite this, questions remain about the exact behavior of water, particularly at low temperatures. Researchers probed films of amorphous solid water to observe proton exchange at 114–134 K, temperatures at which water is deeply supercooled. They found that the proton concentration was not uniform across the entire film. Instead, the distribution decayed with distance from the film’s platinum substrate where the protons originate. The team was able to use this concentration gradient and the rate of hydrogen–deuterium exchange to determine the proton diffusion constant.
The Impact
The mechanistic details of how protons move within water are not fully understood, despite water’s ubiquity. There is debate about whether a significant energy barrier to diffusion is even present. By measuring the energy barrier and coefficient for diffusion, researchers can gain insight into the mechanism of proton diffusion at low temperatures. The data support the hypothesis that proton diffusion requires making changes to the hydrogen bonding network, presenting a clear energy barrier. This mechanistic knowledge may also enhance scientific understanding of chemical reactions involving supercooled water in the upper troposphere, which affect atmospheric composition, precipitation, and lightning formation.
Summary
The movement of protons in water drives a wide range of physical, chemical, and biological processes. Despite its prevalence, scientists still do not fully understand the mechanism behind proton diffusion in water. A research team studied supercooled water films ~100 nm thick on a platinum substrate at 114–134 K. They specifically probed the exchange of protons with deuterated water, watching the conversion of D2O to HOD. Unexpectedly, the team found that the protons were unevenly distributed across the film. This nonuniformity is caused by attraction between the protons and negative charges remaining at the platinum surface. The spatial distribution matched established theory for other analogous systems, allowing the researchers to identify the proton concentration. The proton concentration and D2O decay were then used to calculate the proton diffusion coefficient. This work is the first report of a measured proton diffusion coefficient in supercooled water at 114–134 K, showing that the protons migrate ~100-times faster than the water molecules. The temperature dependence of the diffusion suggests that the hydrogen bonding network needs to be rearranged for diffusion to occur, providing insight into the diffusion mechanism.
Contact
Greg Kimmel, Pacific Northwest National Laboratory, greg.kimmel@pnnl.gov
Megan Dunlap, Pacific Northwest National Laboratory, megan.dunlap@pnnl.gov
Funding
This work was supported by the Department of Energy, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences, and Biosciences, Condensed Phase and Interfacial Molecular Science program, FWP 16248.
Published: June 2, 2025
Dunlap, M. K., Kringle, L., Kay, B. D., Kimmel, G. A. 2024. “Proton diffusion and hydrogen/deuterium exchange in amorphous solid water at temperatures from 114 to 134 K,” J. Chem. Phys. 161, 244504. DOI: 10.1063/5.0233755