Leveraging Curvature on Nitrogen-Doped Carbon Materials for Hydrogen Storage
Identifying how curvature affects the doping and hydrogen binding energies of carbon-based materials provides a framework for designing hydrogen storage materials
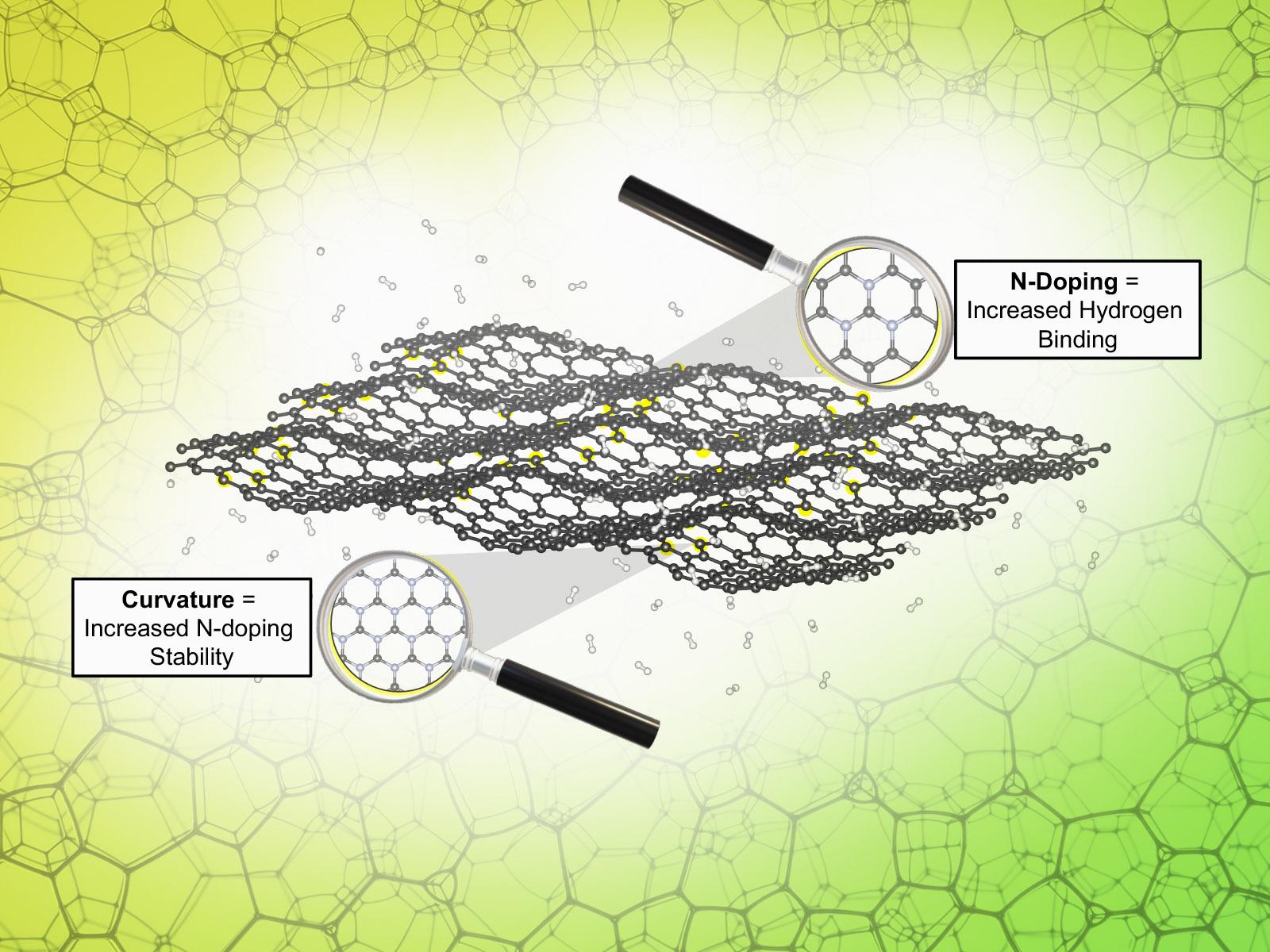
The electronic effects resulting from nitrogen dopants preferentially aggregating on curved sites in 2D carbon materials have a synergistic effect on the hydrogen binding energies.
(Image by Peter S. Rice | Pacific Northwest National Laboratory)
The Science
Carbon-based materials, such as graphene, show promise for storing hydrogen to enable large-scale energy storage. Graphene, when modified with atoms like nitrogen, has the potential to store H2 at room temperature. Researchers used computational methods to develop insight into how the combination of material topology and the presence of dopants affected the binding energy of hydrogen in 2D carbon-based materials. They show that the nitrogen atoms aggregate on curved sites and modulate the materials’ propensity to store hydrogen.
The Impact
As both global energy demands and the effects of climate change increase, finding fossil fuel free energy sources remains an urgent challenge. Clean H2, produced by water electrolysis from renewable resources, is an attractive alternative to store energy but is challenging to transport as a pure liquid or gas. Finding pathways to bind and store hydrogen reversibly in a solid or liquid carrier is a significant area of research. The results of this work will provide a framework for researchers designing carbon-based materials for hydrogen storage, helping accelerate the creation of effective solutions for H2 transport.
Summary
Researchers used density functional theory calculations to study suitability of doped and undoped carbon-based materials for hydrogen storage. The results demonstrate that both the nitrogen dopant concentration and system curvature can significantly influence the hydrogen storage properties of these materials. π-orbital axis vector analysis shows that the lattice curvature affects the doping stability and hydrogen binding strength by altering/tuning the shape of the active site. Curved sites in 2D carbon materials are the thermodynamically favored location for nitrogen dopants to integrate. Therefore, curved sites with more nitrogen atoms present will have the optimum H binding energy for reversible storage. Designing materials for H2 storage devices must strongly consider active site configuration and local electronic effects rather than strain engineering.
PNNL Contact
Tom Autrey, Pacific Northwest National Laboratory, tom.autrey@pnnl.gov
Bojana Ginovska, Pacific Northwest National Laboratory, bojana.ginovska@pnnl.gov
Funding
This work was performed at Pacific Northwest National Laboratory (PNNL) and supported by the Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering, Physical Behavior of Materials Program, PNNL FWP 80110. PNNL is operated by Battelle for DOE under Contract No. DE-AC05-76RL01830. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science user facility located at Lawrence Berkeley National Laboratory, operated under Contract No. DEAC02-05CH11231. G. L. acknowledges the DOE Office of Science Community College Internship Program for support.
Published: February 1, 2024
Rice P. S., G. Lee, B. Schwartz, T. Autrey, and B. Ginovska. 2024. “Leveraging Curvature on N-Doped Carbon Materials for Hydrogen Storage,” Small. [DOI: 10.1002/smll.202310162]