Designing Protein-Material Interfaces
Reviewing recent advances in protein design for complex materials
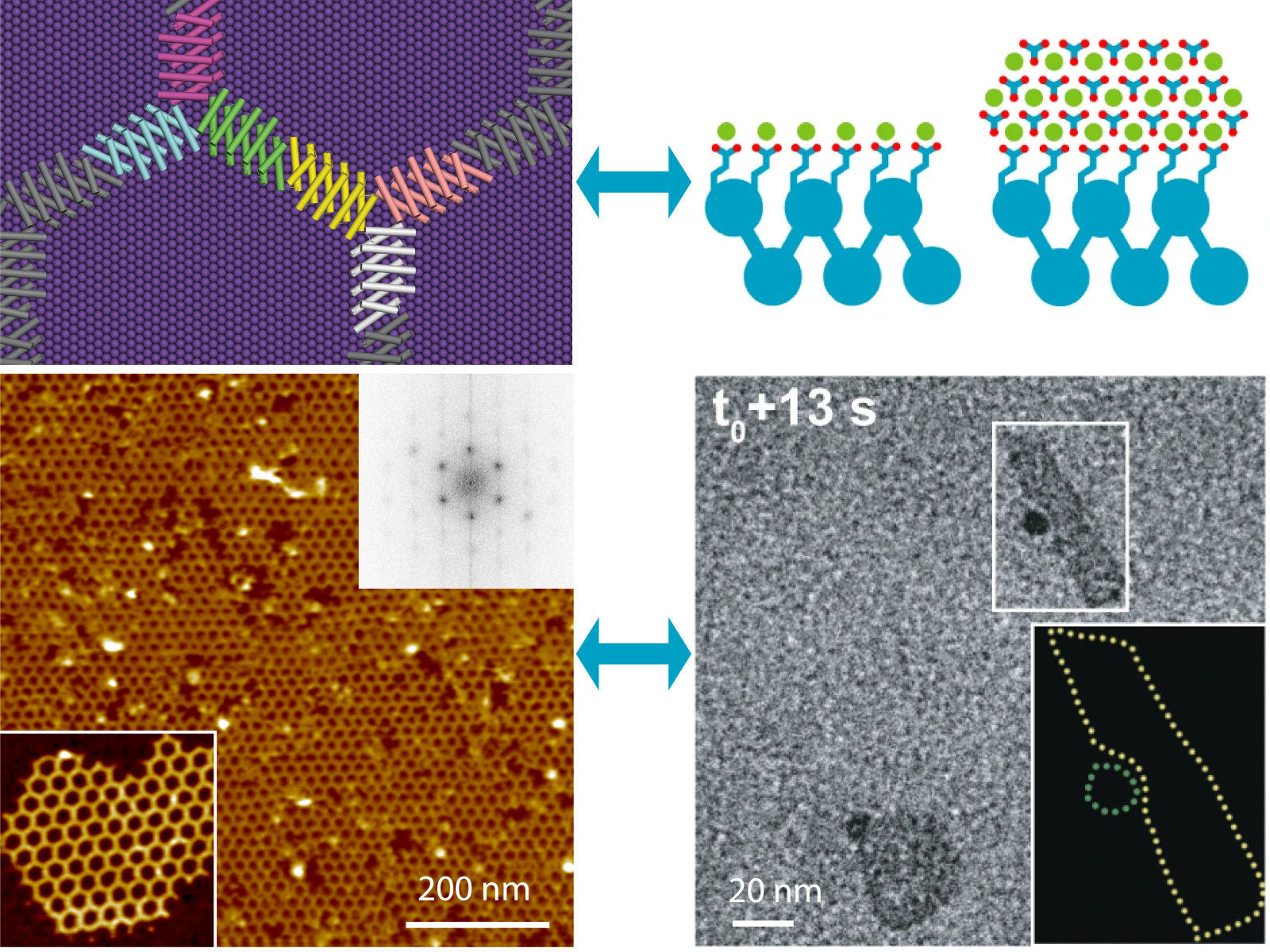
Complex material structures can form through templated crystal growth or direct protein self-assembly across a range of natural and designed proteins.
(Figure by Shuai Zhang | Pacific Northwest National Laboratory; reproduced from s41586-019-1361-6 and s41467-023-43608-1)
Many of the problems facing humanity today have already been solved in some fashion by living creatures. Long-time collaborators at Pacific Northwest National Laboratory (PNNL) and the University of Washington (UW) reviewed recent research advances in human-designed, or de novo, bio-inspired protein-based materials.
The article, authored by PNNL researchers Shuai Zhang and Jim De Yoreo, along with UW’s David Baker and Harley Pyles, is part of a celebration of the 50th anniversary of the publication of the MRS Bulletin. De Yoreo and Baker (the recipient of the 2024 Nobel Prize in Chemistry) presented the Kavli keynote lecture on this topic at the MRS Spring 2025 meeting.
Proteins have a wide range of potential 3D structures and tunable compositions. Combining proteins with other materials can lead to complex, hierarchical structures with novel and specifically targeted properties. These structures can arise from either the self-assembly of proteins on the materials or through protein-directed growth of materials.
To understand these processes, researchers use in situ imaging and spectroscopy techniques that allow them to watch materials form in real time, gaining valuable insights into growth mechanisms. While this approach has been broadly applied to naturally occurring proteins, the team has also applied these techniques to systems with de novo proteins.
“Understanding how these complicated materials form requires a combined approach, bridging modeling and advanced characterization techniques,” said Zhang. “As we continue increasing the resolution, spatial and temporal, of our in situ methods, we keep filling in the details of growth processes.”
They showed that complex material structures can form through templated crystal growth or direct protein self-assembly across a range of natural and designed proteins. The specific path primarily depends on the protein structure, with minor modifications having significant impacts. The flexibility of de novo proteins offers increased tunability for material-protein interactions, potentially leading to more targeted applications across industries.
“I think that the future of hybrid materials is bright,” said De Yoreo. “We’ve made an immense amount of progress toward understanding how these systems behave, but there’s still more to learn.”
Published: January 16, 2026