Quantifying the Molecular Interactions of Alcohols in Zeolite Pores
A comprehensive investigation provides quantitative data on the interaction between zeolite pores and linear alcohols, with hydroxyl group interactions playing the largest role
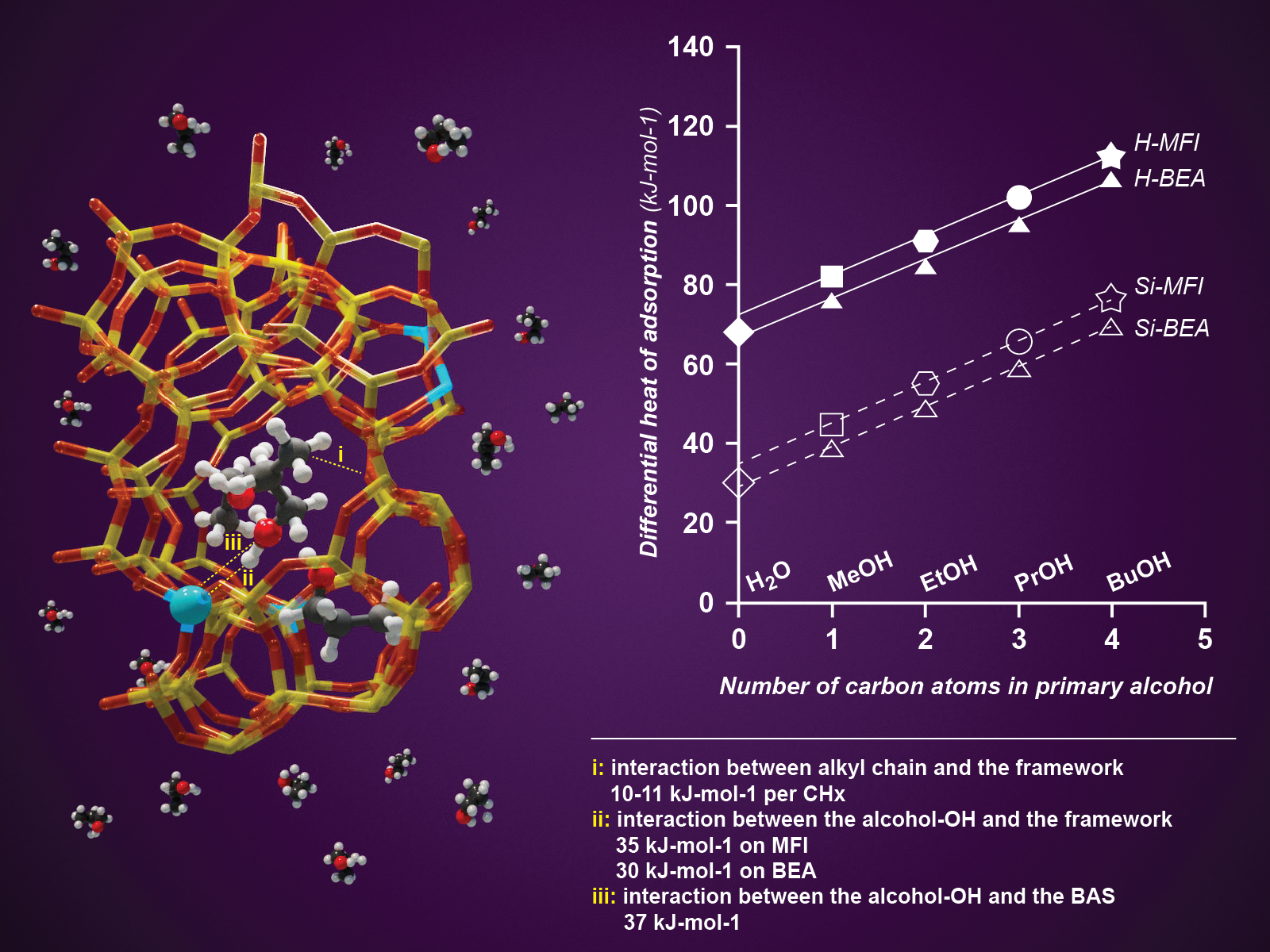
Researchers quantified three main interaction types between short chain alcohols and zeolite pore walls.
(Image by Cortland Johnson | Pacific Northwest National Laboratory)
The Science
Quantifying the fundamental interactions between molecules and the walls of zeolite pores is essential to designing better zeolite-based catalysts and sorbents. However, teasing out values for the individual types of interactions is challenging due to the number of factors at play. Researchers systematically studied primary alcohols with increasing numbers of alkyl groups. They were able to quantify three main interaction types between the alcohols and the zeolite pore walls. From weakest to strongest, the interactions were: between the walls and alkyl groups, between the alcohol hydroxyl groups and the pore walls, and between the alcohol hydroxyl groups and the Brønsted acid sites inside the pores.
The Impact
Zeolites are commonly used as catalysts and sorbents due to their well-defined pores and high overall stability. The specific composition and structure of the pores can be tuned, offering opportunities for additional catalyst design. By providing precise and quantitative insights into fundamental interactions governing how polar and nonpolar molecules behave in zeolite pores, this research can facilitate the rational design of more efficient and selective zeolite-based catalysts and sorbents.
Summary
Understanding the quantitative interactions among zeolite pore walls, Brønsted acid sites, and molecules with both polar and nonpolar regions is essential for determining the potential of various zeolites as sorbents and catalysts. Purely siliceous zeolites are known to be hydrophobic, preferentially adsorbing organic molecules even in aqueous environments. To characterize these interactions, researchers used a series of primary alcohols and quantified the specific interactions of the alkyl (CHx) and OH groups in the confined pore space. Three types of interactions were experimentally identified and computationally verified: (1) alkyl groups interacting with the zeolite pore walls (approximately 10 kJ mol–1 per carbon), (2) alcohol OH groups interacting with the pore walls (30–35 kJ mol–1), and (3) alcohol OH groups interacting with Brønsted acid sites (37 kJ mol–1). The team inferred the contribution of the alkyl CHx groups from the incremental increase in sorption enthalpy that occurred as molecular weight increased. They determined the interaction strength of the OH groups by extrapolating the global adsorption enthalpy of the alcohols to a hypothetical OH group without any alkyl groups, a value identical to the adsorption enthalpy of water. The experiments found that water was the only molecule with an adsorption enthalpy lower than its condensation enthalpy on zeolite pore walls, the underlying cause that limits the concentration of water that can be adsorbed.
Contact
Zdenek Dohnalek, Pacific Northwest National Laboratory, zdenek.dohnalek@pnnl.gov
Sungmin Kim, Pacific Northwest National Laboratory, sungmin.kim@pnnl.gov
Funding
This work was supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences program (BES), Division of Chemical Sciences, Geosciences and Biosciences (Advancing key catalytic reaction steps for achieving carbon neutrality, FWP 47319). V.-A.G. and R.R. acknowledge partial support from BES at Oak Ridge National Laboratory (ORNL). ORNL is operated by UT-Battelle under contract no. DE-AC05-00OR22725 for the DOE.
Published: September 24, 2025
Zhao, R., S. Kim, M.-S. Lee, B. A. Jackson, F. Deng, X. Chen, C. Zhou, K. Khivantsev, Y. Liu, V.-A. Glezakou, R. Rousseau, J. A. Lercher. J. Am. Chem. Soc. 147, 26049 (2025). [DOI: 10.1021/jacs.5c09340].