Mastering Tailored Design of Aluminum Nanomaterials
New methodology is cost-effective and more environmentally friendly than other approaches
Published: March 23, 2020
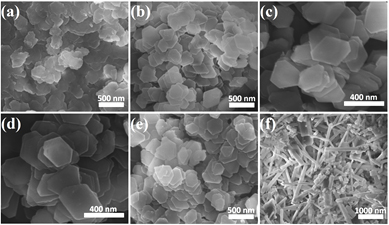
Whether for energy applications or nuclear waste management, industrial processing of aluminum requires understanding its behavior in highly alkaline solutions. Processing slurries and precipitates (typically gibbsite, α-Al(OH)3) from these solutions is aided by controlling the shape of tiny particles that are produced. Researchers at the IDREAM Energy Frontier Research Center, funded by DOE's Office of Science, Basic Energy Sciences, developed a synthesis route. The scientists devised the route based on simple, rational design principles. With it, the team produced highly uniform gibbsite nanoplates with optimal yield.
Why It Matters: Gibbsite is an important ore of aluminum. The ore is processed on an industrial scale in applications ranging from transportation to energy transmission to high-level radioactive waste treatment. Typical processing is energy intensive. The team's work provides a methodology that is cost-effective and more environmentally friendly than other approaches.
Summary: Gibbsite (α-Al(OH)3) is an important natural and industrial material that is used in a wide variety of energy applications, and is a significant component of some of the high-level nuclear waste stored in large quantities at the Hanford Site, Washington, U.S.A., and at the Savannah River Site, South Carolina, U.S.A. Industrial-scale processing of these materials requires an understanding of their behavior in highly alkaline solutions (often called Bayer liquors); processing of slurries and precipitates from these liquors is facilitated by controlling the nanoparticulate gibbsite morphology. The IDREAM team has developed a hydrothermal inorganic synthesis route that is based on simple, rational design principles, and leads to highly uniform hexagonal nanoplates within a basal plane diameter range of 200 to 400 nm. Synchrotron-based x-ray absorption spectroscopy for both aluminum and oxygen reveals that the aluminum coordination in the ideal material is a distorted octahedral geometry with oxygen atoms at two, discrete distances from the central aluminum atom.
Acknowledgments
Sponsors: This work was supported by IDREAM (Interfacial Dynamics in Radioactive Environments and Materials), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences
User Facilities: Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the U.S. Department of Energy (DOE), Office of Science, Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory; Advanced Light Source, supported by the Director, DOE, Office of Science, Basic Energy Sciences
Research Team: Xin Zhang, Carolyn I. Pearce, B. Layla Mehdi, Sebastien Kerisit, Nigel D. Browning, Sue B. Clark, and Kevin M. Rosso, Pacific Northwest National Laboratory; Xianwen Zhang, Hefei University of Technology; Trent R. Graham, Washington State University; Alpha T. N'Diaye, Lawrence Berkeley National Laboratory
Reference: Zhang Z, X Zhang, TR Graham, CI Pearce, BL Mehdi, AT N'Diaye, S Kerisit, ND Browning, SB Clark, and KM Rosso. 2017. "Fast Synthesis of Gibbsite Nanoplates and Process Optimization using Box-Behnken Experimental Design." Crystal Growth & Design. Article ASAP. DOI: 10.1021/acs.cgd.7b01400
Published: March 23, 2020