Atmospher Sci & Global Chg
Research Highlights
October 2016
Imaging Results Are Ones for the Books
In situ real-time imaging now possible for solid-liquid interface
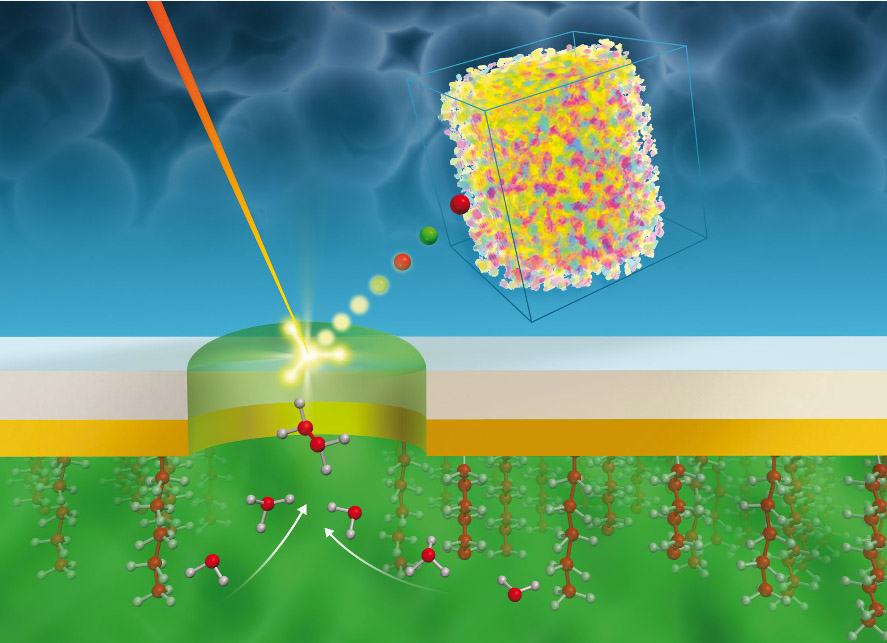
Scientists at Pacific Northwest National Laboratory discovered transient species and reaction pathways in a common electrochemical system interface using liquid secondary ion mass spectrometry and a vacuum-compatible electrochemical microfluidic reactor. Such capabilities are timely in furthering understanding of solid-electrolyte interface phenomena not covered in textbooks Enlarge Image.
Results: Scientists now have new insights into solid-liquid interface phenomena that go far beyond the textbook description. Researchers at Pacific Northwest National Laboratory developed a way to measure this common electrochemical system interface in place and in real time—a previously impossible task. Their work was showcased in the September 21 issue of Chemical Communications and featured on the journal's back cover.
Why It Matters: The solid-liquid interface is the most common interface in electrochemical systems. It consists of a layer of atoms or molecules on an electrode and a layer of adsorbed solvent and species. This interface impacts areas as diverse as prolonging lithium-ion battery life, designing catalytic reactions that can convert biomass to biofuels, and extracellular electron transfer in microbial communities where bacteria catalyze electrode surfaces and shuttle electrons externally, as in a microbial fuel cell.
Being able to explain the reaction mechanism of the solid-liquid interface is critical to underpin its energetics, kinetics, and reactivity and contributes to a new understanding of mass and electron transfer at the interface. However, understanding the workings of such interfaces has been stalled because of their complexity, the difficulty of observing them directly, and lack of true in situ analysis tools—until now.
Methods: The team invented its own electrochemical cell based on microfluidics. "Our cell is vacuum compatible, so we can use it in vacuum surface instruments like time-of-flight secondary ion mass spectrometry (SIMS)," said Dr. Xiao-Ying Yu, a physical chemist who led the study. Using the liquid SIMS technique and the newly patented electrochemical System for Analysis at the Liquid Vacuum Interface (SALVI), the researchers discovered transient species and reaction pathways in the solid-liquid interface.
Notably, this wasn't the first effort to image this interface. In 2014, Yu and colleagues published the first in situ imaging results of the solid electrode and liquid electrolyte interface in Lab on a Chip.
"Our paper got quite a few citations," Yu said. "However, while a recent review reported the advances we have made, the reviewers also did not believe that we could do true in situ measurements. Their perception arises from these factors: a) people do not fully understand the dynamic liquid SIMS technique, and b) we need to provide more illustration of how in situ imaging of the buried solid-liquid interface can be captured using the in situ liquid secondary SIMS technique enabled by SALVI."
So the PNNL team turned to a textbook example of the solid-liquid interface: a potassium iodide electrolyte and a gold working electrode.
Yu explained: "At the beginning, our aim was to illustrate that our unique approach can do real-time imaging of the solid-liquid interface under dynamic conditions. That's why we chose a system that is well known and thoroughly studied in the literature. However, our results were quite unexpected. While confirming what was known in textbooks, our observations provide new insights about the solid electrode and liquid electrolyte interface that we had not thought about before."
What's Next? Because they can now follow transient species formed as a result of electron transfer among communities in real time, Yu and her team are using this approach to study how microbial communities communicate with one another. In addition, they are applying the approach to do in situ imaging of mass and charge transfer in materials sciences.
Acknowledgments
Sponsors: Support for this work was provided by PNNL's Laboratory Directed Research and Development (LDRD) program through the Chemical Imaging Initiative and an open call LDRD and PNNL's fast deployment program. Research was performed in the Environmental Molecular Sciences Laboratory (EMSL) sponsored by the U.S. Department of Energy, Office of Biological and Environmental Research. U.S. patent 9274059 B2 of the electrochemical microreactor has been issued.
Research Team: Jiachao Yu, Yufan Zhou, Xin Hua, Songqin Liu, Zihua Zhu, and Xiao-Ying Yu, PNNL
User Facility: EMSL
Research Area: Climate and Earth Systems Science
Reference: Yu J, Y Zhou, X Hua, S Liu, Z Zhu, and X-Y Yu. 2016. "Capturing the Transient Species at the Electrode-Electrolyte Interface by In Situ Dynamic Molecular Imaging." Chemical Communications 52(73):10952-10955. DOI: 10.1039/C6CC02893D (article) and 10.1039/C6CC90407F (back cover).
Related Highlights:
PNNL-led Science Team Pioneers Approach to Study Cells at Molecular Level
