PNNL Scientists Work To Understand Alzheimer’s, Degenerative Brain Disorders
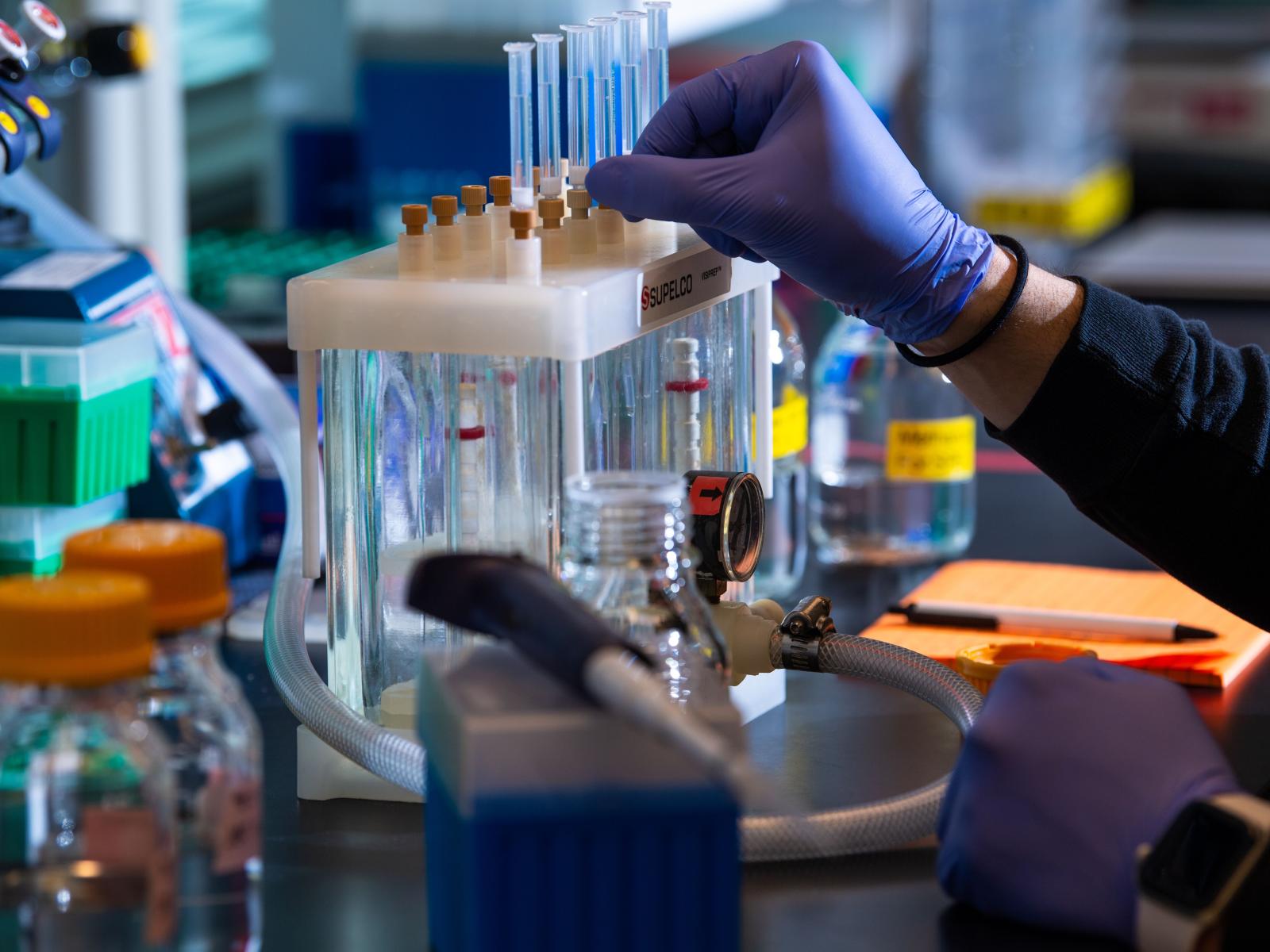
Scientists at PNNL are studying human brain tissue samples, looking for molecular-level patterns that may help them understand what causes the progression of dementia in Alzheimer’s patients.
(Photo by Andrea Starr | Pacific Northwest National Laboratory)
Nearly 120 years after Alois Alzheimer reported what he learned from studying the brain of a patient who suffered from symptoms of what we now call “Alzheimer’s disease,” researchers are still struggling to understand the disease and how it impacts brain function.
At the Department of Energy’s Pacific Northwest National Laboratory, scientists are studying the proteins involved in degenerative brain disorders, such as Alzheimer’s, to help unravel these mysteries.
Their field of research, called proteomics, focuses on exploring the interaction, function, composition and structures of proteins. And their discoveries could ultimately make it easier to diagnose, treat and prevent this and other diseases.
For example, we don’t know why some people whose brains have neuropathologic signs of Alzheimer’s can continue functioning normally while others suffer the debilitating effects of dementia.
As part of a collaborative network involving medical schools across the nation, scientists at PNNL are analyzing blood samples and brain tissue previously collected from more than 1,800 people. Their study seeks to identify molecular-level patterns from large data sets to understand what causes the progression of dementia.
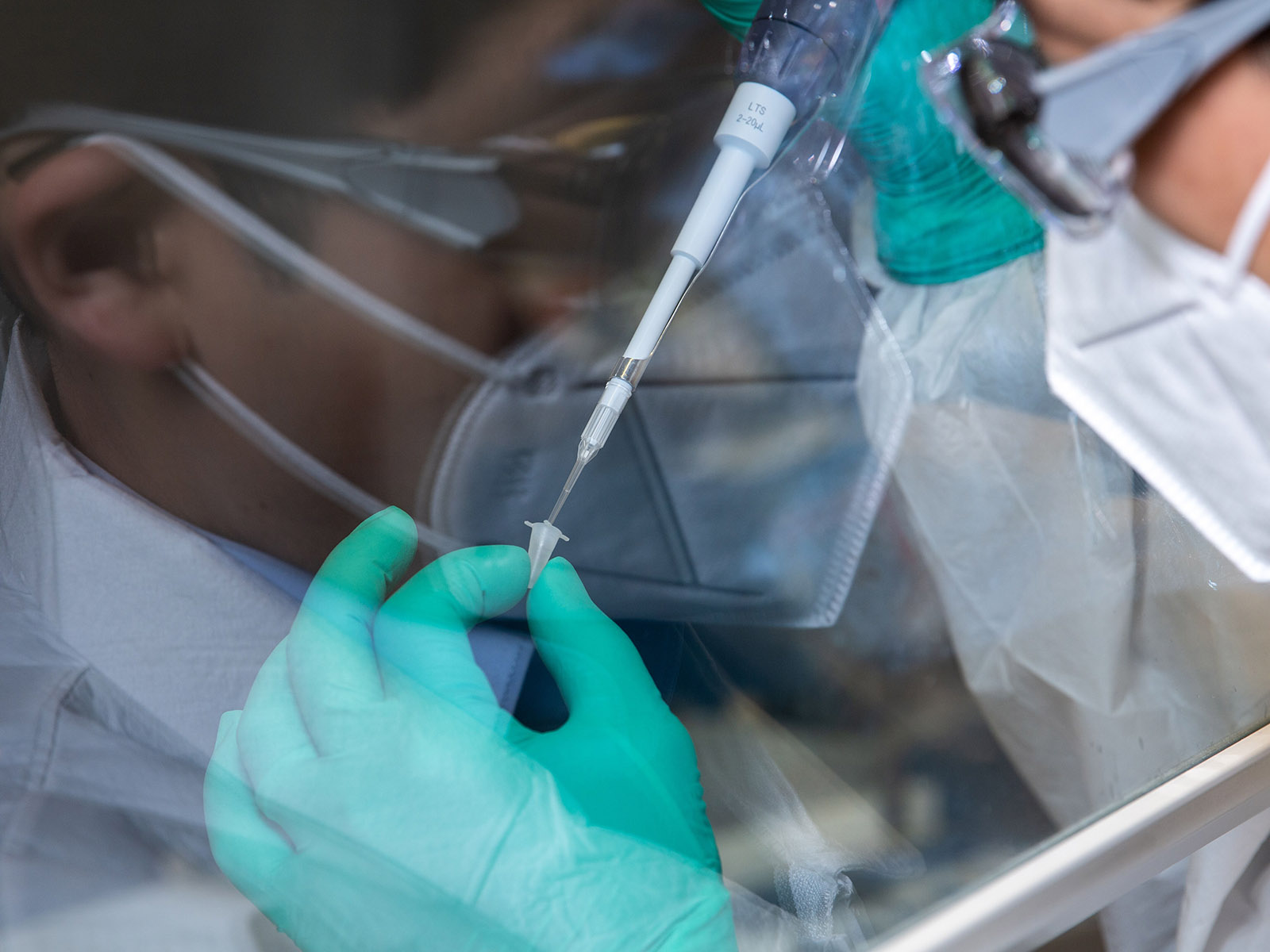
More specifically, they are working to identify the proteins that play a role in the brain’s ability to ward off the impacts of the disease, known as cognitive resilience.
Studying proteins could provide invaluable clues to understanding diseases because these complex molecules control nearly every aspect of cellular activity. Nearly 20,000 proteins are at work every day to keep us alive, each made from hundreds of chemical building blocks.
There is still much to learn about what leads to imperfections as proteins are assembled and how those imperfections contribute to a host of diseases. In Alzheimer’s disease, for example, some symptoms are the result of changes in protein shapes that prevent them from functioning correctly.
Our researchers and their collaborators generated a molecular-level profile for each of the 1,800 patients and the progression of their dementia. Analyzing those profiles, scientists identified three distinct patterns of changes in the proteins and other molecules in the patients’ brains and blood. These patterns could help predict the severity of the disease and future symptoms. Their research also shed light on genetic factors that could predispose individuals to these patterns.
By learning more about which patients had higher cognitive resilience and why, research like this is helping advance potential Alzheimer’s treatments that target the specific proteins that help the brain forestall dementia.
This study relied on PNNL’s computational capabilities for analyzing large data sets and our proteomics capabilities, including powerful scientific instruments that are used to generate that data. Among the most significant are the mass spectrometers at EMSL, the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility at PNNL. Scientists use mass spectrometers to identify and characterize proteins.
For instance, there is a certain brain protein, beta-amyloid, that clumps together and deposits in the brains of Alzheimer’s patients, though no one knows why. These deposits, called plaques, were first discovered by Alzheimer and initially thought to be the reason for deteriorating brain function.
However, trials that target beta-amyloid have failed, implying the picture is more complicated. The true role of this protein fragment is yet to be understood.
In one study, the team examined different forms of beta-amyloid in more than 1,000 brains donated for research. Taking a close look at only those with no evidence of plaque deposits, they discovered a correlation between high levels of a soluble form of the same protein and the rate of cognitive decline.
These results suggest that other mechanisms involving beta-amyloid impact cognition.
Fundamental research like this at PNNL is helping us understand the complex inner workings of Alzheimer’ and other diseases. I am inspired by those who advance scientific discovery—and how their contributions give us hope for longer, healthier lives.
Steven Ashby, director of Pacific Northwest National Laboratory, writes this column monthly. To read previous Director's Columns, please visit our Director's Column Archive.
Published: February 20, 2023